
AgileDoc® SLC
System and Product Lifecycle ManagementImport
AgileDoc imports system and product lifecycle information into a change control database.
Organize
Information such as process control strategies and product recipes is organized and documented according to configured object models.
Report
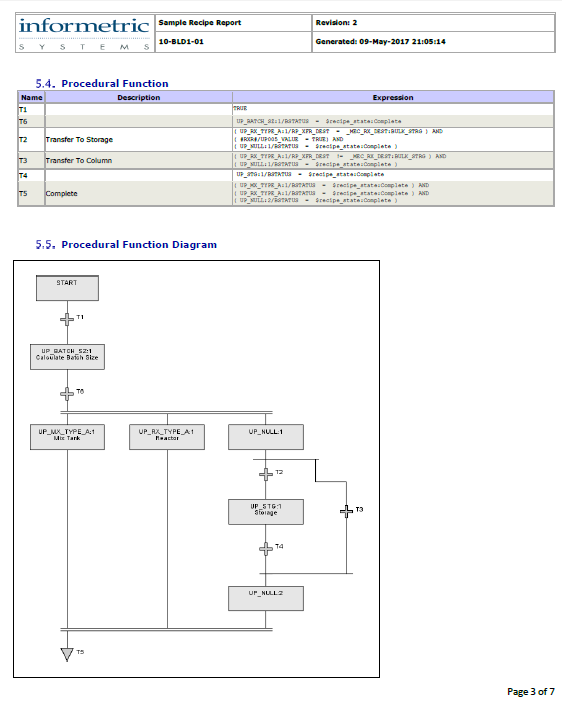
AgileDoc reports can encompass virtually every aspect of a system.
Writing and maintaining lifecycle documentation can consume most of a project’s schedule and budget – and still result in inconsistent and error-riddled documents. Traditionally, documents were first created in a word processing environment, then iteratively reconciled with the actual implementation during development and deployment, and finally maintained using time-intensive write/review/edit/approve/revise cycles.
AgileDoc® streamlines documentation life cycle management by automatically generating documents from imported and archived information. SLC documentation can be generated from manufacturing automation and information systems, thus greatly reducing the effort associated with cGMP documentation while increasing accuracy.
Documentation is further simplified by the AgileDoc Standard Template library. Click on ADS Templates below to learn more.
Key Benefits
Detect coding errors early
Minimize the number of deviations that arise during system validation
Automate time-consuming, manually intensive software documentation tasks
Substantially reduce system deployment cost and compress project timelines
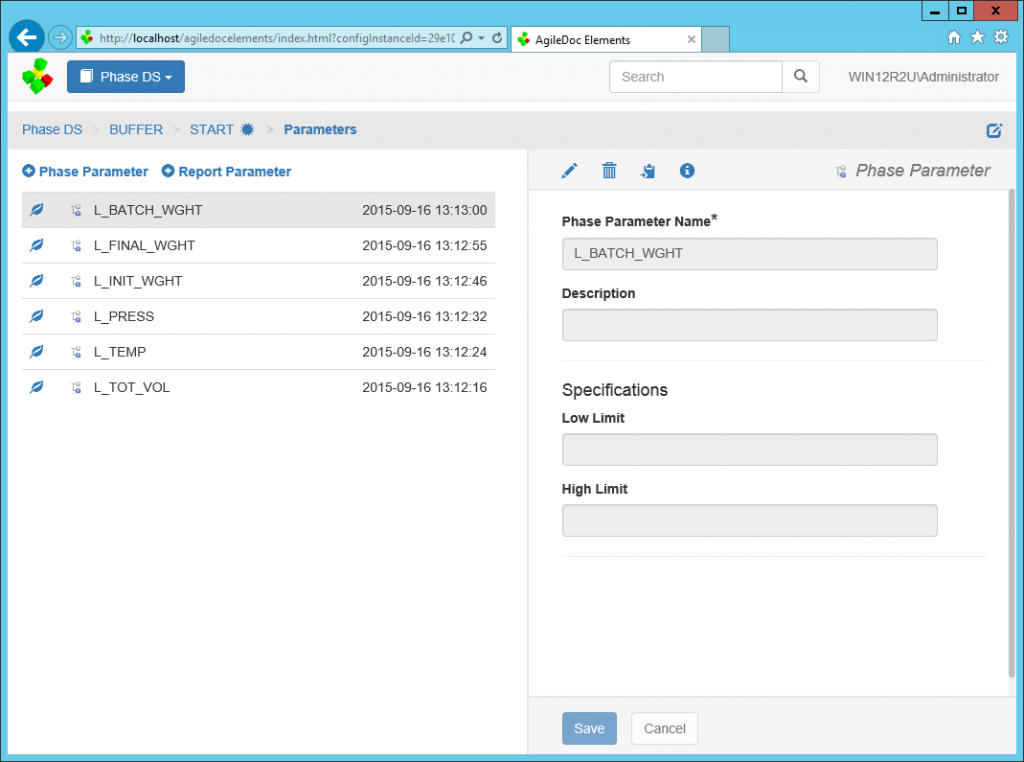
AgileDoc® Elements
While the configuration associated with manufacturing automation and information systems is controlled with AgileDoc, change control metadata and annotation associated with documenting these systems can be managed through AgileDoc® Elements and automatically linked to relevant system modules. AgileDoc Elements ensures data integrity by managing information in a secure, configurable environment.

